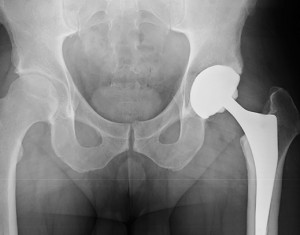
700 Sue Metal-on-Metal Hip Manufacturers in Britain
July 4, 2016Seven hundred Britains have filed a massive metal-on-metal hip implant lawsuit against three makers of the dangerously defective implants. The defendants in the case reportedly include: Zimmer, which made the Durom cup implant; DePuy, the Johnson & Johnson subsidiary that made the Pinnacle system; and Corin, which manufactured the Cormet hip replacement system. Metal-on-metal hip implants...